13. Liquids, Solids & Intermolecular Forces
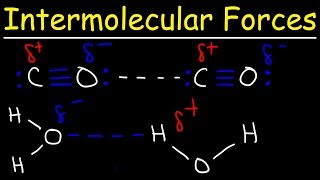
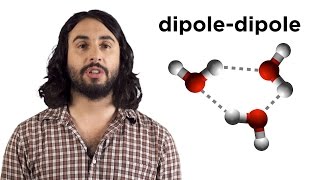
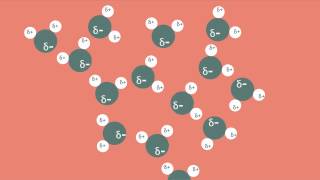
Intermolecular Forces

Get help from an AI Tutor
Ask a question to get started.
Problem 31
Textbook Question
Textbook QuestionA number of salts containing the tetrahedral polyatomic anion, BF4-, are ionic liquids, whereas salts containing the somewhat larger tetrahedral ion SO42 - do not form ionic liquids. Explain this observation.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
504
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos