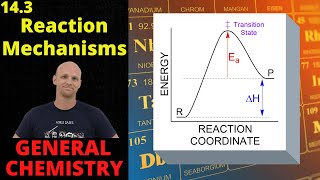
15. Chemical Kinetics
Reaction Mechanism

Get help from an AI Tutor
Ask a question to get started.
Problem 72b
Textbook Question
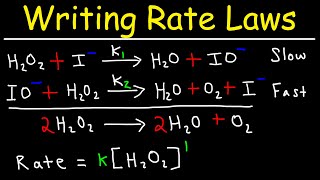
Textbook QuestionThe decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought to proceed by a two-step mechanism: H2O21aq2 + I - 1aq2¡H2O1l2 + IO- 1aq2 1slow2 IO- 1aq2 + H2O21aq2¡H2O1l2 + O21g2 + I - 1aq2 1fast2 (a) Write the chemical equation for the overall process.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
443
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos