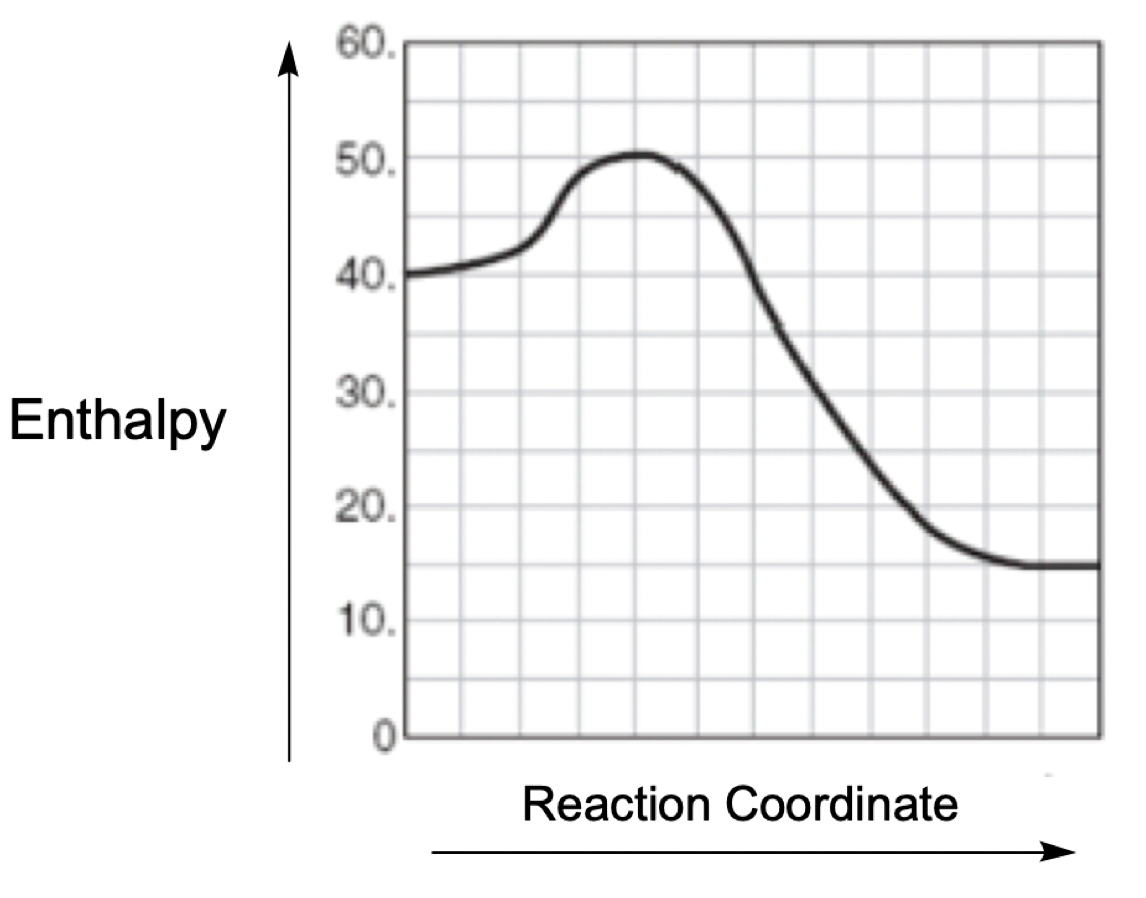
A catalyst is a substance that accelerates a chemical reaction by lowering the activation energy required for the reaction to occur, without being consumed in the process. In an energy diagram, the reactants are represented at the beginning, while the products are at the end. The difference in energy between these two points indicates the overall change in energy of the reaction.
Within the energy diagram, the highest point of the curve represents the transition state, which is crucial for understanding the activation energy. The activation energy (denoted as \(E_a\)) is the energy difference between the reactants and the transition state. In an uncatalyzed reaction, the activation energy is relatively high, as illustrated by the red curve, which we can label as \(E_{a \, \text{uncat}}\).
When a catalyst is introduced, the activation energy decreases, as shown by the blue curve, which represents the catalyzed reaction. This lower activation energy (denoted as \(E_{a \, \text{cat}}\)) allows the reactants to more easily reach the transition state and convert into products. Consequently, a lower activation energy results in a faster reaction rate, as the reactants can more readily overcome the energy barrier represented by the transition state.
In summary, the role of a catalyst is to facilitate the conversion of reactants to products by reducing the activation energy, thereby increasing the reaction rate. This fundamental concept is essential in understanding how catalysts function in various chemical processes.