6. Chemical Quantities & Aqueous Reactions
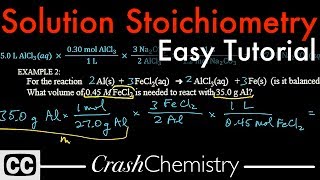
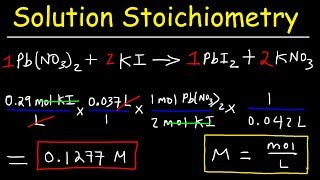
Solution Stoichiometry

Get help from an AI Tutor
Ask a question to get started.
Problem 110
Textbook Question
Textbook QuestionAmmonium chloride, NH4Cl, is a very soluble salt in water. (d) How many grams of silver nitrate do you need to add to the solution in part (c) to precipitate all of the chloride as silver chloride?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
847
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos