- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

In cellular biochemistry, organic reactions can be basically grouped as addition, elimination, or substitution. Using this classification, how would you describe the reaction below?

(i) Provide the structure of the alkene reactant that was used to make the following product:

(ii) Identify the other reagent required for the reaction to occur.
What is the correct name for the product of the hydrogenation of 3,5-dimethyl-2-hexene?
Provide the structure of the substance formed from the given reaction below:

Predict the product of hydrogenation of 3-ethylhex-3-ene in the presence of PtO2, a platinum catalyst.
Farnesene is the compound responsible for the characteristic smells of green apples. Its IUPAC name is 3,7,11-trimethyl-dodeca-1,3,6,10-tetraene. Predict the major product formed when farnesene reacts with excess HBr.
Sketch the products of the addition reaction of 3-ethyl-2-methylpent-1-ene with HBr and identify the major and the minor products.
(i) Drawthe structure of the alkene reactant that was used to make the following product:

(ii) Identify the other reagent required for the reaction to occur.
Give the structure of the compound formed in the reaction given below:

What is the starting material needed for the hydration reaction below?
Sketch the structure that corresponds to each IUPAC name:
I. 2-Chlorophenol
II. 2-Aminobenzoic acid
III. 1,3,5-Benzenetriol
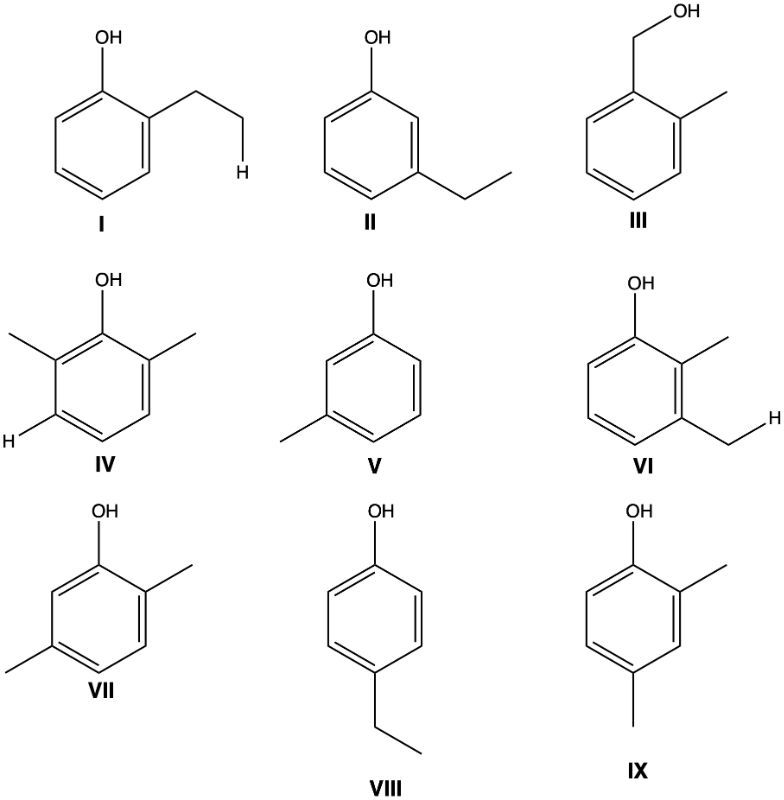
Determine all the isomeric phenols with the formula C8H10O in the given structures.
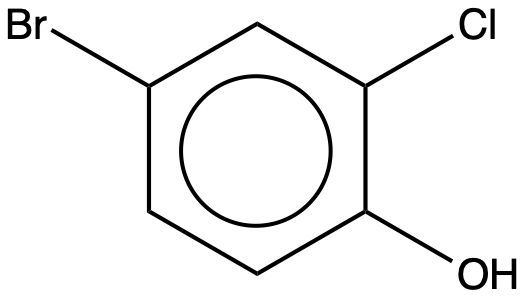
What is the IUPAC name for the given compound?
Picric acid or trinitrophenol (TNP) is widely used in explosives and munitions. Picric is formed from phenol. To avoid side reactions, phenol is first sulfonated to form p-hydroxybenzenesulfonic acid. This is followed by successive nitration. The sulfonic acid group is then displaced by heating in dilute sulfuric acid and final nitration to produce the desired product, picric acid. Considering that nitration only occurs in the ortho and para positions relative to the hydroxy group, predict the structure of picric acid.
Phenols undergo aromatic substitution reactions. Write the chemical equation when p-nitrophenol reacts with Cl2. Note that this reaction will form two substitution products.