The red arrow in the graph (see margin) indicates the changes that occur in the nucleus of an atom during a nuclear reaction. Identify the isotopes involved as product and reactant, and name the type of decay process.
Ch.11 Nuclear Chemistry

Chapter 11, Problem 9
A 1.00 mL sample of red blood cells containing chromium-51 as a tracer was injected into a patient. After several hours, a 5.00 mL sample of blood was drawn and its activity compared to the activity of the injected tracer sample. If the collected sample activity was 0.10% of the original tracer, calculate the total blood volume of the patient (see the Chemistry in Action 'Medical Uses of Radioactivity,' p. 338).
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the problem. The goal is to calculate the total blood volume of the patient using the dilution principle. The activity of the tracer in the collected blood sample is 0.10% of the original tracer activity, and the relationship between the activity and the blood volume can be used to determine the total blood volume.
Step 2: Write the dilution equation. The dilution equation is based on the principle that the total activity of the tracer remains constant. The equation is:
C 1 V 1 C 2 V 2 C 1 V 1 C 2 V 2
Step 3: Express the relationship between the concentrations. Since the activity of the collected sample is 0.10% of the original tracer activity, we can write:
C 2 C 1
Step 4: Rearrange the equation to solve for the total blood volume (V 2 V 2 C 1 V 1 C 2 C 2 C 1 V 2 V 1
Step 5: Substitute the known values into the equation. The volume of the original tracer sample (V 1 V 2

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Radioactive Tracers
Radioactive tracers are substances that emit radiation and are used in medical diagnostics to track biological processes. In this context, chromium-51 serves as a tracer to monitor the distribution and volume of blood in the patient. By measuring the radioactivity of the blood sample, healthcare professionals can infer the total blood volume based on the known activity of the injected tracer.
Recommended video:
Guided course

Measuring Radioactivity Concept 1
Dilution Principle
The dilution principle states that the concentration of a substance decreases as it is distributed in a larger volume. In this scenario, the activity of the tracer in the drawn blood sample is a fraction of the original activity, allowing for the calculation of the total blood volume. By knowing the initial volume of the tracer and its activity, one can determine how much blood is present in the patient based on the diluted activity measured.
Recommended video:
Guided course
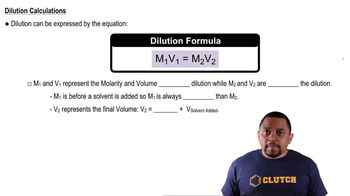
Dilutions
Activity Measurement
Activity measurement refers to the quantification of radioactive decay events occurring in a sample over time, typically expressed in counts per minute (CPM) or becquerels (Bq). In this case, the activity of the blood sample is compared to the original tracer's activity to assess how much of the tracer remains in the bloodstream. This comparison is crucial for calculating the total blood volume based on the proportion of tracer activity detected.
Recommended video:
Guided course

Measuring Radioactivity Concept 1
Related Practice
Textbook Question
1479
views
Textbook Question
A β-emitting radiation source gives 250 units of radiation at a distance of 4.0 m. At what distance does the radiation drop to one-tenth its original value?
1485
views
1
rank
Textbook Question
A solution of selenium-75, a radioisotope used in the diagnosis of pancreatic disease, is found just prior to administration to have an activity of 44 μCi/mL. If 3.98 mL were delivered intravenously to the patient, what dose of Se-75 (in μCi) did the patient receive?
2120
views
Textbook Question
A typical chest X ray exposes a patient to an effective dose of 0.02 mSv. How many rem is this, and how many chest X rays would a patient have to receive before biological effects would be observed? (The limit from Table 11.6 is >25 rem.)
1595
views
