An element has completely filled n = 1 and n = 2 shells and has six electrons in the n = 3 shell. Identify the element and its major group (i.e., main group, transition, etc.). Is it a metal or a nonmetal? Identify the orbital in which the last electron is found.
Ch.2 Atoms and the Periodic Table

Chapter 2, Problem 30
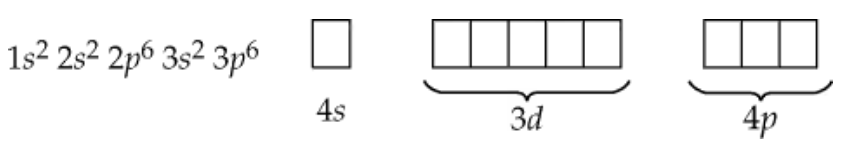
Use the following orbital-filling diagram to show the electron configuration for As:
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the problem. The goal is to determine the electron configuration for arsenic (As) using the orbital-filling diagram. Arsenic has an atomic number of 33, meaning it has 33 electrons to distribute among its orbitals.
Step 2: Recall the order of orbital filling based on the Aufbau principle. Electrons fill orbitals in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p. Each orbital has a specific capacity: s orbitals hold 2 electrons, p orbitals hold 6, and d orbitals hold 10.
Step 3: Begin filling the orbitals. Start with the 1s orbital and continue filling each subsequent orbital until all 33 electrons are distributed. Use the Pauli exclusion principle (no more than 2 electrons per orbital with opposite spins) and Hund's rule (electrons fill degenerate orbitals singly before pairing).
Step 4: Write the electron configuration. After filling the orbitals, the electron configuration for arsenic will be written in the form of subshells with superscripts indicating the number of electrons in each subshell. For example, 1s², 2s², etc.
Step 5: Verify the configuration. Ensure the total number of electrons adds up to 33 and that the configuration matches the orbital-filling diagram. The final configuration should reflect the correct distribution of electrons in the orbitals, including the partially filled 4p subshell for arsenic.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's orbitals. It is represented using a notation that indicates the energy levels and sublevels occupied by electrons, following the Aufbau principle, Pauli exclusion principle, and Hund's rule. For example, the electron configuration for arsenic (As) is 1s² 2s² 2p⁶ 3s² 3p³, which shows how its 33 electrons are arranged.
Recommended video:
Guided course
The Electron Configuration: Condensed
Orbital Filling Diagram
An orbital filling diagram visually represents how electrons fill atomic orbitals according to specific rules. It typically uses boxes or lines to represent orbitals and arrows to indicate electrons, with their spins. This diagram helps in understanding the order of filling and the maximum number of electrons each orbital can hold, which is crucial for determining the electron configuration of elements like arsenic.
Recommended video:
Guided course

Energy Diagrams Example 1
Periodic Table Trends
The periodic table organizes elements based on their atomic number and electron configurations, revealing trends in properties such as electronegativity, ionization energy, and atomic radius. Understanding these trends is essential for predicting the behavior of elements, including arsenic, which is located in group 15 and exhibits properties characteristic of metalloids. This context aids in grasping why arsenic has a specific electron configuration.
Recommended video:
Guided course

Periodic Trend: Metallic Character
Related Practice
Textbook Question
1860
views
Textbook Question
For chlorine, identify the group number, give the number of electrons in each occupied shell, and write its valence-shell configuration.
1620
views
Textbook Question
Use the following blank periodic table to show where the elements matching the following descriptions appear.
a. Elements with the valence-shell electron configuration ns2 np5
b. An element whose third shell contains two p electrons
c. Elements with a completely filled valence shell
2300
views
Textbook Question
How do atoms of different elements differ?
1976
views
Textbook Question
Find the mass in atomic mass units of the following:
a. 1 O atom, with a mass of 2.66 × 10-23 g
b. 1 Br atom, with a mass of 1.31 × 10-22 g
1667
views
Textbook Question
How many O atoms of mass 15.99 amu are in 15.99 g of oxygen?
1539
views
