Consider the following Lewis symbols for elements X and Y:
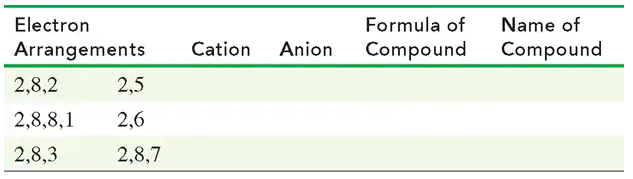
c. What ions would be formed by X and Y?

 Verified step by step guidance
Verified step by step guidance



Consider the following Lewis symbols for elements X and Y:
c. What ions would be formed by X and Y?
Consider the following Lewis symbols for elements X and Y:
d. What would be the formula of a compound of X and Y?
Consider the following Lewis symbols for elements X and Y:
e. What would be the formula of a compound of X and sulfur?
State the number of valence electrons, bonding pairs, and lone pairs in each of the following Lewis structures:
c.
Match each of the Lewis structures (a to c) with the correct diagram (1 to 3) of its shape, and name the shape; indicate if each molecule is polar or nonpolar. Assume X and Y are nonmetals and all bonds are polar covalent.
<IMAGE>
c.
Match each of the formulas (a to c) with the correct diagram (1 to 3) of its shape, and name the shape; indicate if each molecule is polar or nonpolar.
<IMAGE>
a. PBr3