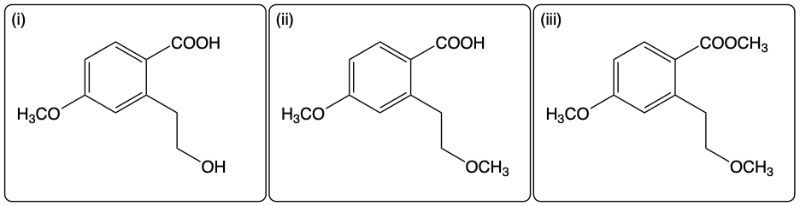
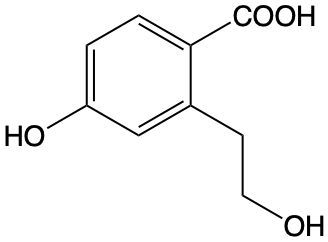
The reactions involve the deprotonation and methylation of the hydroxyl groups. The first NaH equivalent will remove the most acidic proton from the phenolic group, the second NaH equivalent will remove the primary alcohol proton, and the third equivalent will remove the proton from the carboxylic group.