Introduction to Chemical Bonding exam Flashcards
 Back
BackTerms in this set (29)
Chemical Bond
Attractive force that holds atoms together to form molecules and compounds.
What is a molecule?
A substance that contains at least two chemically bound atoms.
Compound
A molecule that contains at least two different elements.
Is O2 a molecule or a compound?
O2 is a molecule but not a compound.
Intramolecular Bonds
Interactions between atoms within the same molecule.
What is an example of a compound?
Water (H2O) is an example of a compound.
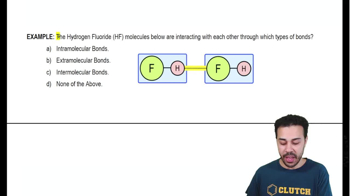
Intermolecular Bonds
Interactions between atoms of different molecules.
Chemical Formula
Represents the number and type of atoms in a molecule or compound.
What does C6H12O6 represent?
The chemical formula for glucose.
What is the difference between intramolecular and intermolecular bonds?
Intramolecular bonds are within the same molecule, while intermolecular bonds are between different molecules.
What does the 'comp' in compound imply?
It implies that compounds are more complex, containing at least two different elements.
What is the chemical formula for water?
H2O
What is glucose?
A sugar compound with the chemical formula C6H12O6.
What are the elements in water?
Hydrogen and Oxygen.
What is the significance of chemical formulas?
They provide a quick way to represent the structure of molecules and compounds.
What does H2O stand for?
Two hydrogen atoms and one oxygen atom.
What is an example of an intermolecular bond?
The hydrogen bond between two different water molecules.
What does the term 'molecule' include?
Any substance with at least two chemically bound atoms, regardless of whether they are the same or different elements.
What is the difference between molecules and compounds?
All compounds are molecules, but not all molecules are compounds.
What is an example of a molecule that is not a compound?
Oxygen gas (O2).
What does the chemical formula C6H12O6 indicate?
6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
What is the role of chemical bonds?
To hold atoms together to form molecules and compounds.
What is the difference between H2O and O2?
H2O is a compound and a molecule, while O2 is only a molecule.
What are the two types of chemical bonds discussed?
Intramolecular bonds and intermolecular bonds.
What does the 'intra' in intramolecular bonds remind you of?
It reminds you that these bonds are 'trapped' within the same molecule.
What is the significance of intermolecular bonds?
They are interactions between different molecules, important for understanding molecular interactions.
What is the chemical formula for glucose?
C6H12O6
What does the term 'inter' in intermolecular bonds signify?
It signifies interactions between different molecules.
What is the purpose of a chemical formula?
To succinctly represent the composition of molecules and compounds.