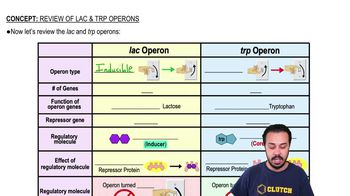
Textbook Question
X-gal is a colorless, lactose-like molecule that can be split into two fragments by ββ-galactosidase. One of these product molecules creates a blue color. The photograph here shows E. coli colonies growing in a medium that contains X-gal. Find three colonies whose cells have functioning copies of ββ-galactosidase. Find three colonies whose cells might have mutations in the lacZ or the lacY genes. Suppose you analyze the protein-coding sequence of the lacZ and lacY genes of cells from the three mutant colonies and find that these sequences are wild type (normal). What other region of the lac operon might be altered to account for the mutant phenotype of these colonies?
1298
views