15. Chemical Kinetics
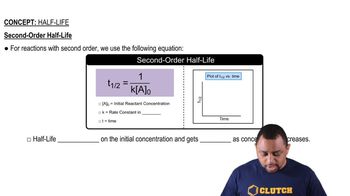
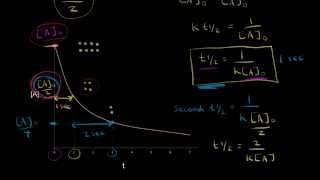
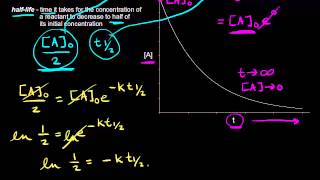
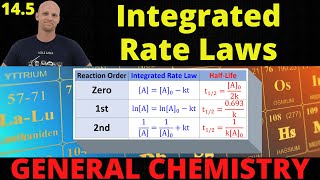
Half-Life

Get help from an AI Tutor
Ask a question to get started.
Problem 44
Textbook Question
Textbook QuestionThe first-order rate constant for the decomposition of N2O5, 2 N2O51g2¡4 NO21g2 + O21g2, a t 70 C i s 6.82 * 10-3 s-1. Suppose we start with 0.0250 mol of N2O51g2 in a volume of 2.0 L. (c) What is the half-life of N2O5 at 70 C ?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
832
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos