15. Chemical Kinetics
Integrated Rate Law

Get help from an AI Tutor
Ask a question to get started.
Problem 101
Textbook Question
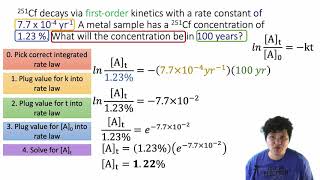
Textbook QuestionThe rate of a first-order reaction is followed by spectroscopy, monitoring the absorbance of a colored reactant at 520 nm. The reaction occurs in a 1.00-cm sample cell, and the only colored species in the reaction has an extinction coefficient of 5.60 * 103 M-1 cm-1 at 520 nm. (d) How long does it take for the absorbance to fall to 0.100?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
808
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos