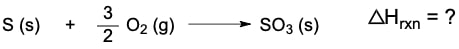
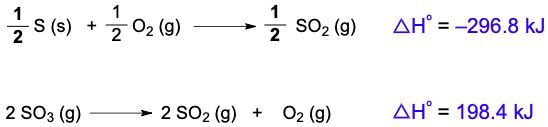
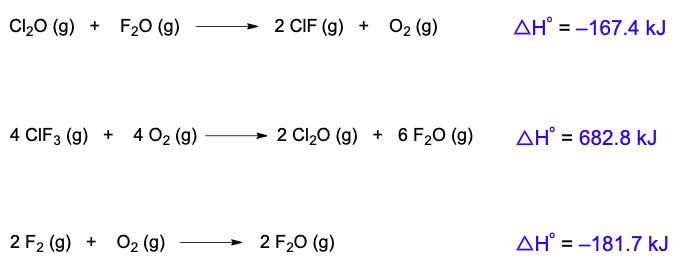
Hess's law is a fundamental principle in thermochemistry that allows us to determine the overall enthalpy change of a reaction by rearranging thermochemical equations. A thermochemical equation is a chemical equation that includes the enthalpy of reaction, denoted as ΔHrxn. The key concept is that any modification to the original equation will directly affect the enthalpy of reaction.
For example, consider the thermochemical equation:
2 Mg(s) + O2(g) → 2 MgO(s) with ΔHrxn = -1204 kJ.
There are three primary operations that can be performed on this equation, each influencing ΔHrxn accordingly:
1. **Multiplying the Equation**: If the entire equation is multiplied by a factor, the coefficients of the reactants and products change, and the enthalpy change must also be multiplied by the same factor. For instance, if we multiply the equation by 2, it becomes:
4 Mg(s) + 2 O2(g) → 4 MgO(s) with ΔHrxn = -2408 kJ.
2. **Dividing the Equation**: Similarly, if the equation is divided by a number, the coefficients are halved, and the enthalpy change is divided by the same number. Dividing the original equation by 2 results in:
1 Mg(s) + 1/2 O2(g) → 1 MgO(s) with ΔHrxn = -602 kJ.
3. **Reversing the Equation**: When the reaction is reversed, the products become reactants and vice versa. This operation also reverses the sign of ΔHrxn. For example, reversing the original equation gives:
2 MgO(s) → 2 Mg(s) + O2(g) with ΔHrxn = +1204 kJ.
In summary, Hess's law emphasizes that the enthalpy of reaction is directly proportional to the changes made to the thermochemical equation. Understanding these relationships is crucial for calculating the enthalpy changes in various chemical reactions.