6. Chemical Quantities & Aqueous Reactions
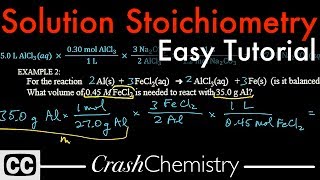
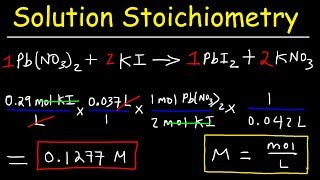
Solution Stoichiometry

Get help from an AI Tutor
Ask a question to get started.
Problem 96
Textbook Question
Textbook QuestionThe commercial production of nitric acid involves the following chemical reactions: 4 NH31g2 + 5 O21g2¡4 NO1g2 + 6 H2O1g2 2 NO1g2 + O21g2¡2 NO21g2 3 NO21g2 + H2O1l2¡2 HNO31aq2 + NO1g2 (c) How many grams of ammonia must you start with to make 1000.0 L of a 0.150 M aqueous solution of nitric acid? Assume all the reactions give 100% yield.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
948
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos