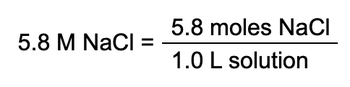Molarity, often denoted as M, is a key concept in chemistry that quantifies the concentration of a solute in a solution. It specifically measures the number of moles of solute per liter of solution, providing a precise way to express concentration. The formula for calculating molarity is given by:
M = \(\frac{\text{moles of solute}}{\text{liters of solution}}\)
In this equation, the numerator represents the amount of solute measured in moles, while the denominator indicates the volume of the solution in liters. Understanding molarity is crucial for solving various problems in chemistry, as it allows for the determination of how much solute is present in a given volume of solution. This concept is often used interchangeably with concentration, but it is important to note that molarity provides a more specific measurement.
As you explore problems related to molarity, you will encounter scenarios that require you to calculate the molarity of different solutions or analyze the components that contribute to molarity. Mastering this concept will enhance your ability to work with solutions in chemical reactions and various laboratory settings.