6. Chemical Quantities & Aqueous Reactions
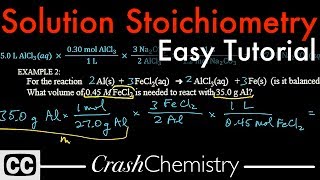
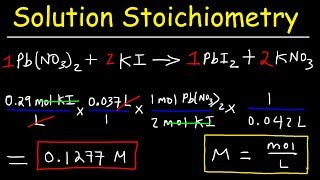
Solution Stoichiometry
Problem 53
Textbook Question
Textbook QuestionDescribe how you would prepare each of the following aqueous solutions, starting with solid KBr: (d) a 0.150 M solution of KBr that contains just enough KBr to precipitate 16.0 g of AgBr from a solution containing 0.480 mol of AgNO3.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
682
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos