17. Acid and Base Equilibrium
Ka and Kb

Get help from an AI Tutor
Ask a question to get started.
Problem 82
Textbook Question
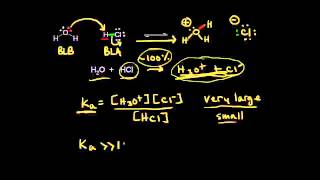
Textbook QuestionPyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (b) Using Appendix D, calculate the Ka for pyridinium bromide.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
413
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos