14. Solutions
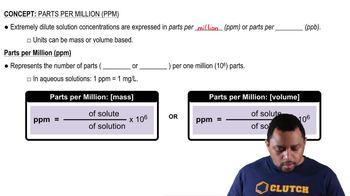
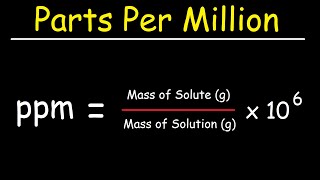
Parts per Million (ppm)

Get help from an AI Tutor
Ask a question to get started.
Problem 115a
Textbook Question
Textbook QuestionFederal regulations set an upper limit of 50 parts per million (ppm) of NH3 in the air in a work environment [that is, 50 molecules of NH31g2 for every million molecules in the air]. Air from a manufacturing operation was drawn through a solution containing 1.00 * 102 mL of 0.0105 M HCl. The NH3 reacts with HCl according to: NH31aq2 + HCl1aq2¡NH4Cl1aq2 After drawing air through the acid solution for 10.0 min at a rate of 10.0 L/min, the acid was titrated. The remaining acid needed 13.1 mL of 0.0588 M NaOH to reach the equivalence point. (b) How many ppm of NH3 were in the air? (Air has a density of 1.20 g/L and an average molar mass of 29.0 g>mol under the conditions of the experiment.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
325
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos