Textbook Question
Order the following atoms according to increasing atomic radius: Rb, Cl, As, K.
1099
views

 Verified step by step guidance
Verified step by step guidance


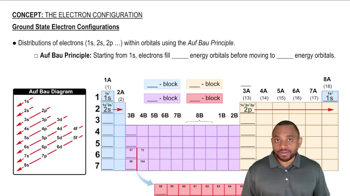
Order the following atoms according to increasing atomic radius: Rb, Cl, As, K.
Imagine a universe in which the four quantum numbers can have the same possible values as in our universe except that the angular-momentum quantum number l can have integral values of 0, 1, 2...n + 1 (instead of 0, 1, 2..., n - 1). (c) Draw an orbital-filling diagram for the element with atomic number 12.