Textbook Question
Write equations for the loss of an electron by a K atom and the gain of an electron by a K+ ion.
1471
views

 Verified step by step guidance
Verified step by step guidance
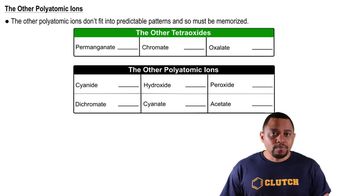


Write equations for the loss of an electron by a K atom and the gain of an electron by a K+ ion.
What is the difference between an acid and a base?
Write equations to show how the substances listed in Problem 3.75 give ions when dissolved in water.
a. H2CO3
b. HCN
c. Mg(OH)2
d. KOH