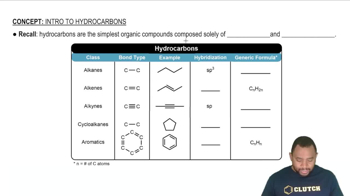
Saturation and Unsaturation
Saturation refers to hydrocarbons that contain only single bonds between carbon atoms, allowing for the maximum number of hydrogen atoms. Unsaturated hydrocarbons, on the other hand, contain double or triple bonds, which reduce the number of hydrogen atoms that can bond with the carbon backbone. Identifying whether a hydrocarbon is saturated or unsaturated is essential for calculating the required hydrogen atoms.

 Verified step by step guidance
Verified step by step guidance