Textbook Question
According to the aufbau principle, which orbital is filled immediately after each of the following in a multielectron atom?(a) 4s (b) 3d(c) 5f(d) 5p
764
views

 Verified step by step guidance
Verified step by step guidance

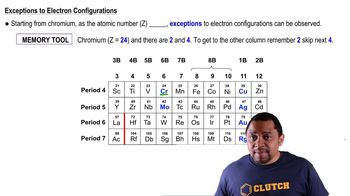
Give the expected ground-state electron configurations for atoms with the following atomic numbers. (a) Z = 55 (b) Z = 40 (c) Z = 80 (d) Z = 62
Draw orbital-filling diagrams for atoms with the following atomic numbers. Show each electron as an up or down arrow, and use the abbreviation of the preceding noble gas to represent inner-shell electrons. (a) Z = 25 (b) Z = 56 (c) Z = 28 (d) Z = 47