Textbook Question
Give the expected ground-state electron configurations for atoms with the following atomic numbers. (a) Z = 55 (b) Z = 40 (c) Z = 80 (d) Z = 62
612
views

 Verified step by step guidance
Verified step by step guidance

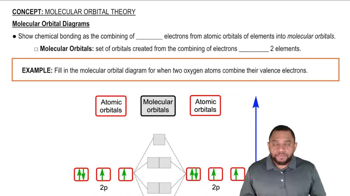

Give the expected ground-state electron configurations for atoms with the following atomic numbers. (a) Z = 55 (b) Z = 40 (c) Z = 80 (d) Z = 62
Write the symbol, give the ground-state electron configuration, and draw an orbital-filling diagram for each of the following atoms. Use the abbreviation of the preceding noble gas to represent the inner-shell electrons. (a) The heaviest alkaline earth metal