Textbook Question
Describe the difference between a Lewis structure and a condensed structure in terms of atoms and bonds shown in the structures.
1197
views

 Verified step by step guidance
Verified step by step guidance
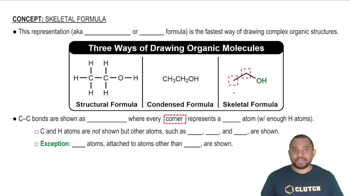


Describe the difference between a Lewis structure and a condensed structure in terms of atoms and bonds shown in the structures.
Draw a skeletal structure for each of the following compounds:
(b)
Draw a skeletal structure for each of the following compounds:
(b)
Draw a Lewis structure for each of the following compounds:
(b)