Textbook Question
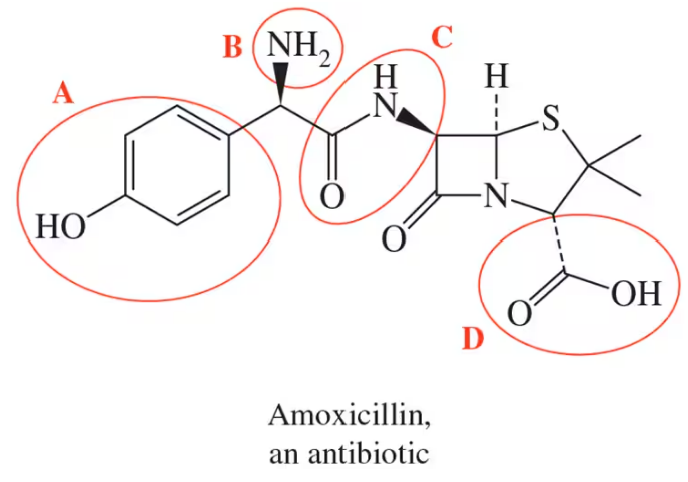
Identify all of the functional groups in each of the following molecules:
(a)
697
views

 Verified step by step guidance
Verified step by step guidance



Identify all of the functional groups in each of the following molecules:
(a)
Identify all of the functional groups in each of the following molecules:
(b)
Identify all of the functional groups in each of the following molecules:
(b)
Write the condensed formula for each of the following molecules:
(b) 1,3-dichloro-3-methylheptane
Draw skeletal structures for each of the following molecules:
(a) ethylcyclopropane
Draw skeletal structures for each of the following molecules:
(b) cis-1-chloro-3-methylcyclohexane