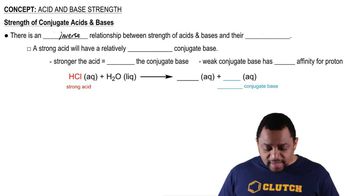
Textbook Question
Write a balanced equation for the proton transfer reaction between hydrofluoric acid (HF) and ammonia (NH3). Identify each conjugate acid-base pair, and rewrite the equilibrium arrows to indicate if the forward or reverse reaction is favored.
1556
views