Textbook Question
What happens when a strong acid such as HBr is dissolved in water?
1970
views

 Verified step by step guidance
Verified step by step guidance
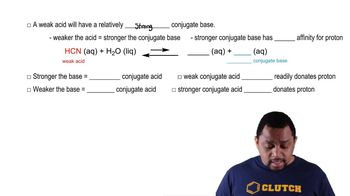


What happens when a strong acid such as HBr is dissolved in water?
What happens when a weak acid such as CH3CO2H is dissolved in water?
What happens when a strong base such as KOH is dissolved in water?
What is the difference between a monoprotic acid and a diprotic acid? Give an example of each.
What is the difference between H+ and H3O+?
How is Kw defined, and what is its numerical value at 25 °C?