Textbook Question
What are likely formulas for the following molecules?
a. CH2Cl?
b. BH?
c. NI?
d. SiCl?
2346
views

 Verified step by step guidance
Verified step by step guidance
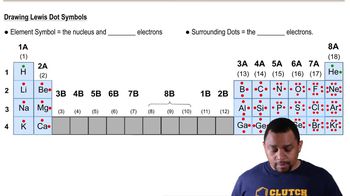

What are likely formulas for the following molecules?
a. CH2Cl?
b. BH?
c. NI?
d. SiCl?
What is a coordinate covalent bond, and how does it differ from a covalent bond?
Identify the bonds formed between the following pairs of atoms as either covalent or ionic.
d. Zinc and fluorine
Which of the following contains a coordinate covalent bond? (Hint: How many covalent bonds would you expect the central atom (underlined/bold) to form?)
a. PbCl2
b. Cu(NH3)42+
c. NH4+
A compound of gallium with chlorine has a melting point of 77°C and a boiling point of 201°C. Is the compound ionic or covalent? What is a likely formula?
Distinguish between the following:
b. A structural formula and a condensed structure