A typical oral rehydration solution (ORS) for infants contains 90 mEq/L Na+, 20 mEq/L K+, 110 mEq/L Cl– and 2.0% (m/v) glucose (MW = 180g/mol).
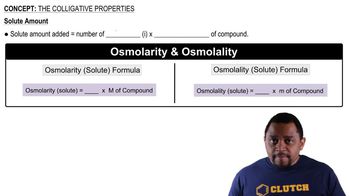
b. What is the osmolarity of the solution, and how does it compare with the osmolarity of blood plasma?

 Verified step by step guidance
Verified step by step guidance
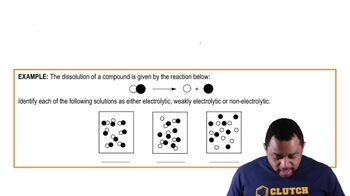

A typical oral rehydration solution (ORS) for infants contains 90 mEq/L Na+, 20 mEq/L K+, 110 mEq/L Cl– and 2.0% (m/v) glucose (MW = 180g/mol).
b. What is the osmolarity of the solution, and how does it compare with the osmolarity of blood plasma?
Assume that two liquids are separated by a semipermeable membrane, with pure solvent on the right side and a solution of a solute on the left side. Make a drawing that shows the situation after equilibrium is reached.
<IMAGE>
When 1 mol of HCl is added to 1 kg of water, the boiling point increases by 1.0 °C, but when 1 mol of acetic acid, CH3CO2H is added to 1 kg of water, the boiling point increases by only 0.5 °C. Explain.
How can you tell a solution from a colloid?
Why does water not dissolve motor oil?
The solubility of NH3 gas in water at an NH₃ pressure of 760.0 mmHg and 25°C is 51.8 g/100 mL and 27.0 g/100 mL at 50°C.
a.What is the solubility of NH3 if its partial pressure is reduced to 225.0 mmHg?