Textbook Question
What alcohols would you oxidize to obtain the following carbonyl compounds?
a.
b.
c.
729
views

 Verified step by step guidance
Verified step by step guidance


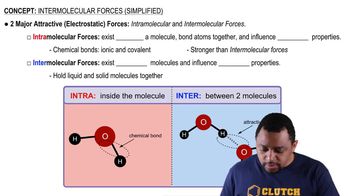
What alcohols would you oxidize to obtain the following carbonyl compounds?
a.
b.
c.
What is the structural relationship between a thiol and an alcohol?
Oxidation of a dithiol such as 2,5-hexanedithiol forms a six-membered ring containing a disulfide group as part of the ring. Draw the structure of this cyclic disulfide (Hint: Draw the starting compound in line structure format first).
Propanol is very soluble in water, but ethanethiol and chloroethane are only slightly soluble. Explain.
Define the following terms:
b. Achiral
Define the following terms:
c. Chiral carbon