'Designer vinegars' have become very popular over the past decade. Vinegars made from champagne, merlot, and other wines are but a few of these. All wines contain ethanol, and these vinegars are simply wines containing microorganisms that have caused oxidation of the ethanol present. If vinegar is simply ethanol that has been oxidized, what is the structure of the acid formed?
Ch.14 Some Compounds with Oxygen, Sulfur, or a Halogen

Chapter 14, Problem 74
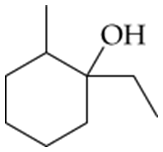
Using the alcohol shown, draw all the possible alkenes that might be formed on its dehydration. Which do you think will be the major product(s)? Which do you think will be the minor product(s)? It is alright to have more than one major and minor product.
 Verified step by step guidance
Verified step by step guidance1
Identify the alcohol functional group in the given molecule. Dehydration of alcohols involves the removal of a water molecule (H₂O), which consists of a hydroxyl group (-OH) and a hydrogen atom from an adjacent carbon atom.
Determine all possible β-hydrogens (hydrogens on carbons adjacent to the carbon bearing the -OH group). Each β-hydrogen can lead to a different alkene product when eliminated.
Apply the E1 (unimolecular elimination) mechanism, which is typical for dehydration of alcohols under acidic conditions. The steps include: (1) protonation of the -OH group to form a better leaving group (H₂O), (2) loss of H₂O to form a carbocation intermediate, and (3) elimination of a β-hydrogen to form an alkene.
Consider carbocation rearrangements if applicable. If the initial carbocation can rearrange to a more stable carbocation (e.g., via hydride or alkyl shifts), this rearrangement will occur before elimination, leading to additional alkene products.
Use Zaitsev's rule to predict the major and minor products. Zaitsev's rule states that the more substituted alkene (the one with more alkyl groups attached to the double-bonded carbons) is generally the major product, while less substituted alkenes are minor products. Draw all possible alkenes and classify them accordingly.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Dehydration Reaction
A dehydration reaction involves the removal of a water molecule from an alcohol, leading to the formation of an alkene. This process typically occurs under acidic conditions, where the alcohol is protonated to form a better leaving group. The resulting carbocation can rearrange or lose a proton to form the double bond characteristic of alkenes.
Recommended video:
Guided course

Alcohol Reactions: Dehydration Reactions Concept 1
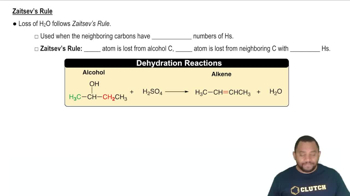
Zaitsev's Rule
Zaitsev's Rule states that in elimination reactions, the more substituted alkene is generally the major product. This is because more substituted alkenes are more stable due to hyperconjugation and the inductive effect. Understanding this rule helps predict which alkenes will be favored in the dehydration of alcohols.
Recommended video:
Guided course
Zaitsev’s Rule Concept 2
Regioselectivity
Regioselectivity refers to the preference of a chemical reaction to yield one structural isomer over others. In the context of dehydration, different alkenes can form depending on the position of the double bond. Analyzing the possible products and their stability allows chemists to determine which will be major or minor products based on their regioselectivity.
Related Practice
Textbook Question
773
views
Textbook Question
Draw all possible cyclic C7H14O alcohol isomers having a cyclohexane ring and a methyl group. (Hint: Adapt the method described in Worked Example 12.12 to arrive at your answers.)
803
views
Textbook Question
Identify all chiral centers in the isomers that you drew for part (a).
729
views
Textbook Question
Using the alcohol shown, draw all the possible alkenes that might be formed on its dehydration. Which alkenes can exist as cis–trans isomers? Draw them, in both condensed and line structure, and identify each as cis or trans. Explain your choices.
730
views
