Textbook Question
Write the formulas and IUPAC names for the following common alcohols.
d. Diol used as antifreeze (two answers)
540
views

 Verified step by step guidance
Verified step by step guidance
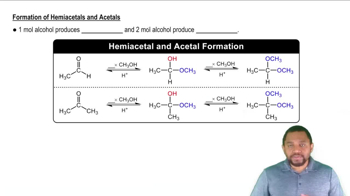
Write the formulas and IUPAC names for the following common alcohols.
d. Diol used as antifreeze (two answers)
Name the following compounds:
c.
d.
Name the following compounds:
e.
f.
Draw all possible cyclic C7H14O alcohol isomers having a cyclohexane ring and a methyl group. (Hint: Adapt the method described in Worked Example 12.12 to arrive at your answers.)
Identify all chiral centers in the isomers that you drew for part (a).
Using the alcohol shown, draw all the possible alkenes that might be formed on its dehydration. Which do you think will be the major product(s)? Which do you think will be the minor product(s)? It is alright to have more than one major and minor product.