What kind of reaction does each of these enzymes catalyze?
c. A reductase

 Verified step by step guidance
Verified step by step guidance


What kind of reaction does each of these enzymes catalyze?
c. A reductase
Alcohol dehydrogenase (ADH) catalyzes the following reaction. To what class of enzymes does ADH belong?
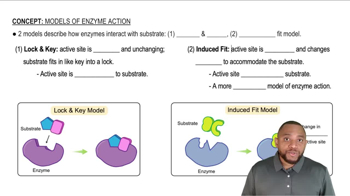
What is the difference between the lock-and-key model of enzyme action and the induced-fit model?
How do you explain the observation that pepsin, a digestive enzyme found in the stomach, has a high catalytic activity at pH 1.5, while trypsin, an enzyme of the small intestine, has no activity at pH 1.5?
What general effects would you expect the following changes to have on the rate of an enzyme-catalyzed reaction for an enzyme that has its maximum activity at body temperature (about 37°C)?
a. Raising the temperature from 37°C to 70°C
What general effects would you expect the following changes to have on the rate of an enzyme-catalyzed reaction for an enzyme that has its maximum activity at body temperature (about 37°C)?
c. Adding an organic solvent, such as methanol