Textbook Question
Lipids are not soluble in water. Are lipids polar or nonpolar molecules?
898
views

 Verified step by step guidance
Verified step by step guidance


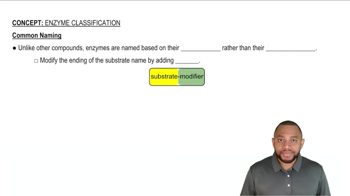
Lipids are not soluble in water. Are lipids polar or nonpolar molecules?
What are some functions of lipids in the body?
Describe some similarities and differences in the structures of a saturated fatty acid and an unsaturated fatty acid.
Stearic acid and linoleic acid each have 18 carbon atoms. Why does stearic acid melt at 69 °C but linoleic acid melts at –5 °C?