Which statements completed with a to e will be true and which will be false?
An atom of N compared to an atom of Li has a larger (greater)
b. ionization energy

 Verified step by step guidance
Verified step by step guidance


Which statements completed with a to e will be true and which will be false?
An atom of N compared to an atom of Li has a larger (greater)
b. ionization energy
Which statements completed with a to e will be true and which will be false?
An atom of N compared to an atom of Li has a larger (greater)
c. number of protons
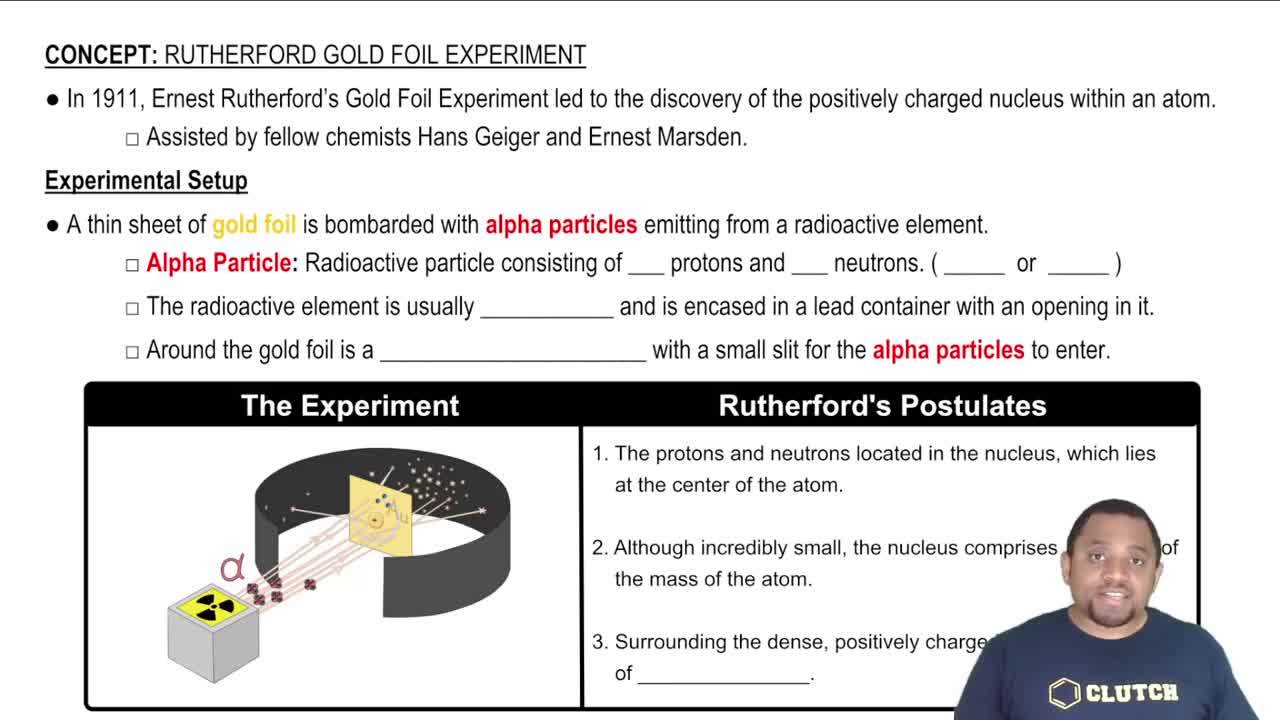
Use Rutherford's gold-foil experiment to answer each of the following:
b. How did the results differ from what he expected?
Match the subatomic particles (1 to 3) to each of the descriptions below:
1. protons
2. neutrons
3. electrons
b. surround the nucleus
Consider the following atoms in which X represents the chemical symbol of the element:
168X 169X 1810X 178X 188X
a. What atoms have the same number of protons?
Consider the following atoms in which X represents the chemical symbol of the element:
168X 169X 1810X 178X 188X
c. Which atoms have the same mass number?