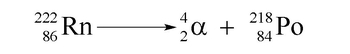
Alpha decay is a type of radioactive decay where an unstable nucleus emits an alpha particle, which consists of 2 protons and 2 neutrons. This process results in a decrease in the atomic mass of the original element by 4 units, as the atomic mass is calculated by the sum of protons and neutrons. The alpha particle can be represented as:
\[\frac{4}{2} \text{He}\]
Here, the alpha particle is equivalent to the element Helium, which has the same atomic mass of 4 and an atomic number of 2.
To illustrate alpha decay, consider the isotope Polonium-210 (represented as Po), which has an atomic mass of 210 and an atomic number of 84. During alpha decay, Polonium-210 emits an alpha particle:
\[\text{Po}_{84}^{210} \rightarrow \text{He}_{2}^{4} + \text{Pb}_{82}^{206}\]
In this reaction, the total atomic mass must be conserved. The original atomic mass of 210 is balanced by the sum of the emitted alpha particle (4) and the new element, Lead-206 (206). Therefore, the new element formed after the decay is Lead-206, which has an atomic number of 82. This ensures that the atomic numbers also balance, as 84 (from Polonium) equals 2 (from Helium) plus 82 (from Lead).
In summary, alpha decay transforms Polonium-210 into Lead-206 while emitting an alpha particle, Helium, as a byproduct. It is crucial to ensure that both atomic mass and atomic number are balanced in nuclear reactions, similar to balancing in chemical reactions.