Textbook Question
According to MO theory, which molecule or ion has the highest bond order? O2, O2- , O22-
640
views

 Tro 4th Edition
Tro 4th Edition Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory
Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory Problem 81
Problem 81 Verified step by step guidance
Verified step by step guidance


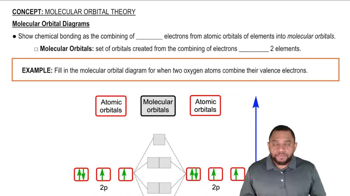
According to MO theory, which molecule or ion has the highest bond order? O2, O2- , O22-
According to MO theory, which molecule or ion has the highest bond energy? O2, O2- , O22-
According to MO theory, which molecule or ion has the shortest bond length? O2, O2- , O22-
Draw an energy diagram for HCl. Predict the bond order and make a sketch of the lowest energy bonding molecular orbital.