The principal component of many kidney stones is calcium oxalate, CaC2O4. A kidney stone recovered from a typical patient contains 8.5 × 1020 formula units of calcium oxalate. How many moles of CaC2O4 are present in this kidney stone? What is the mass of the kidney stone in grams?

Titanium metal is obtained from the mineral rutile, TiO2. The process requires multiple steps, as shown in the following reactions:
TiO2(s) + 2 Cl2(g) + 2 C(s) → TiCl4(s) + 2 CO(g)
TiCl4(s) + 2 Mg(s) → Ti(s) + 2 MgCl2(s)
c. How many kilograms of rutile are needed to produce 95 kg of Ti?
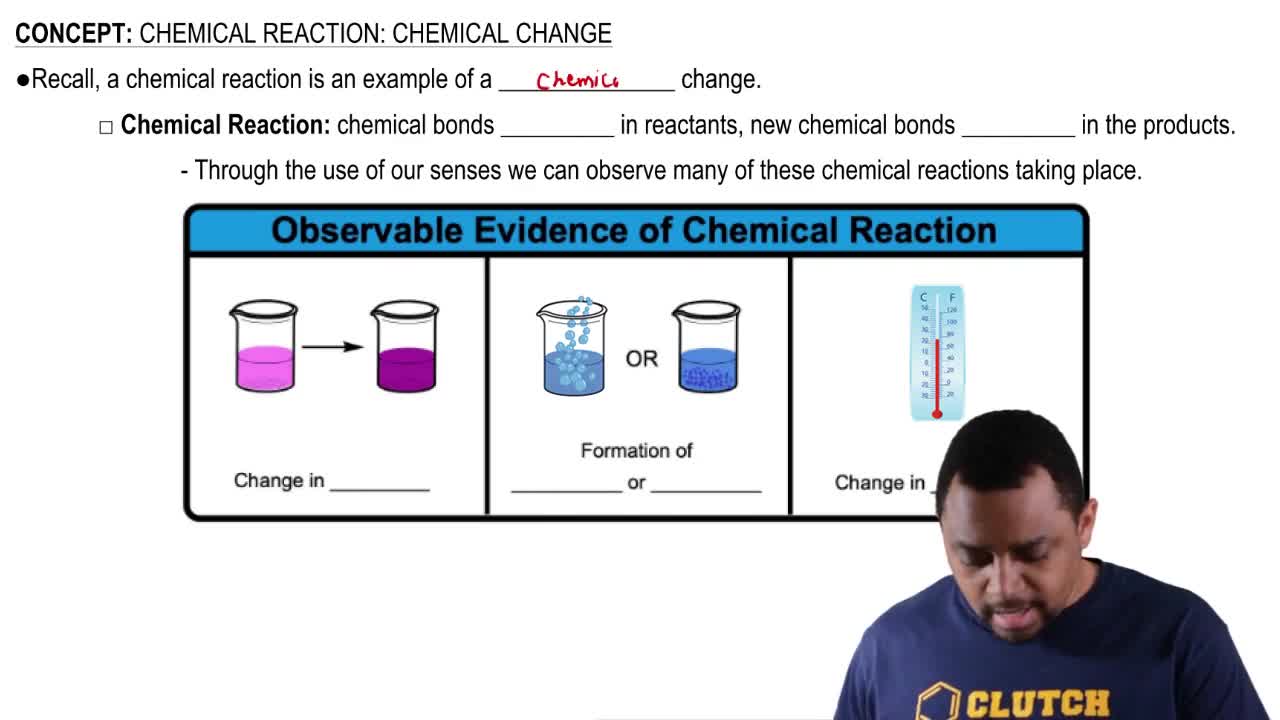
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Stoichiometry

Molar Mass

Chemical Reactions
Titanium metal is obtained from the mineral rutile, TiO2. The process requires multiple steps, as shown in the following reactions:
TiO2(s) + 2 Cl2(g) + 2 C(s) → TiCl4(s) + 2 CO(g)
TiCl4(s) + 2 Mg(s) → Ti(s) + 2 MgCl2(s)
a. Write mole ratios to show the relationship between the reactants and products for each reaction.
Titanium metal is obtained from the mineral rutile, TiO2. The process requires multiple steps, as shown in the following reactions:
TiO2(s) + 2 Cl2(g) + 2 C(s) → TiCl4(s) + 2 CO(g)
TiCl4(s) + 2 Mg(s) → Ti(s) + 2 MgCl2(s)
b. How many moles of TiO2 are needed to form one mole of titanium?
In Problem 6.40, hydrazine reacted with oxygen according to the following (unbalanced) equation: N2H4(l) + O2(g) → NO2(g) + H2O(g)
a. If 75.0 kg of hydrazine are reacted with 75.0 kg of oxygen, which is the limiting reagent?
Nitrobenzene (C6H5NO2) is used in small quantities as a flavoring agent or in perfumes but can be toxic in large amounts. It is produced by reaction of benzene (C6H6) with nitric acid:
C6H6(l) + HNO3(aq) → C6H5NO2(l) + H2O(l)
a. Identify the limiting reagent in the reaction of 27.5 g of nitric acid with 75 g of benzene.
Nitrobenzene (C6H5NO2) is used in small quantities as a flavoring agent or in perfumes but can be toxic in large amounts. It is produced by reaction of benzene (C6H6) with nitric acid:
C6H6(l) + HNO3(aq) → C6H5NO2(l) + H2O(l)
b. Calculate the theoretical yield for this reaction.
