Textbook Question
What is the mass (in amu and in grams) of a single atom of Carbon-12?
2435
views

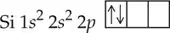
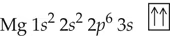
 Verified step by step guidance
Verified step by step guidance


What is the mass (in amu and in grams) of a single atom of Carbon-12?
What is the mass (in grams) of 6.02 × 1023 atoms of Carbon-12?
An unidentified element is found to have an electron configuration by shell of 2 8 18 8 2. To what group and period does this element belong? Is the element a metal or a nonmetal? How many protons does an atom of the element have? What is the name of the element? Write its electron-dot symbol.
Look again at the trends illustrated in Figures 2.3 and 2.4.
a. How do the peaks/valleys correlate with locations in the periodic table?
b. Are there other chemical properties that also exhibit periodic trends? What are they?
<IMAGE>