Classify each of the amino acids in Problem 10.1 as polar (neutral, acidic, or basic) or nonpolar and as hydrophobic or hydrophilic.
a. alanine
b. lysine
c. tryptophan
d. aspartate

 Verified step by step guidance
Verified step by step guidance
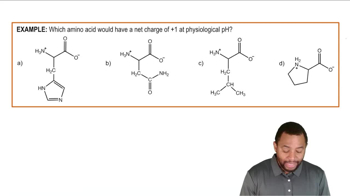


Classify each of the amino acids in Problem 10.1 as polar (neutral, acidic, or basic) or nonpolar and as hydrophobic or hydrophilic.
a. alanine
b. lysine
c. tryptophan
d. aspartate
Give the three-letter and one-letter abbreviations and identify the functional group in the side chain for each amino acid in Problem 10.1.
a. alanine
b. lysine
c. tryptophan
d. aspartate
Draw the structure for each of the following amino acids and put an asterisk (*) next to any chiral carbon centers in your structure:
c. methionine
Draw the structure for the amino acid represented by each of the following abbreviations:
c. Q
Draw the structure for the amino acid represented by each of the following abbreviations:
d. Ile
Draw the structure for the amino acid represented by each of the following abbreviations:
c. Val