Textbook Question
Are the substances shown in italics undergoing oxidation or reduction?
(c) Wine (containing ethanol, CH3CH2OH) sours to vinegar (CH3COOH).
766
views

 Verified step by step guidance
Verified step by step guidance

Are the substances shown in italics undergoing oxidation or reduction?
(c) Wine (containing ethanol, CH3CH2OH) sours to vinegar (CH3COOH).
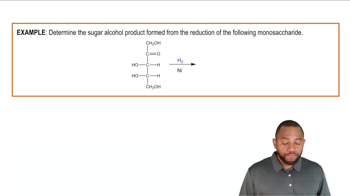
Write the products for the following hydrogenation reactions:
(a)
Write the products for the following hydrogenation reactions:
(c)
Write the products for the following hydrogenation reactions:
(c)