Film badge dosimeters typically include filters to target specific types of radiation. A film badge is constructed that includes a region containing a tin foil filter, a region containing a plastic film filter, and a region with no filter. Which region monitors exposure to α-radiation? Which monitors exposure to β-radiation? Which monitors γ-radiation? Explain.
Ch.11 Nuclear Chemistry

Chapter 11, Problem 86
Thorium-232 decays by a 10-step series, ultimately yielding lead-208. How many α particles and how many β particles are emitted?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the problem. Thorium-232 undergoes a decay series to form lead-208. In this process, α (alpha) particles and β (beta) particles are emitted. Each α particle emission reduces the mass number by 4 and the atomic number by 2, while each β particle emission increases the atomic number by 1 without changing the mass number.
Step 2: Write the nuclear equation for the initial and final isotopes. Thorium-232 (Th-232) has an atomic number of 90, and lead-208 (Pb-208) has an atomic number of 82. The goal is to determine how many α and β particles are emitted during this transformation.
Step 3: Calculate the total change in mass number. The mass number decreases from 232 to 208, which is a difference of 24. Since each α particle reduces the mass number by 4, divide the total change in mass number by 4 to find the number of α particles emitted: \( \text{Number of } \alpha \text{ particles} = \frac{24}{4} \).
Step 4: Calculate the total change in atomic number. The atomic number decreases from 90 to 82, which is a difference of 8. Each α particle reduces the atomic number by 2, so the total reduction in atomic number due to α particles is \( \text{Reduction from } \alpha \text{ particles} = \text{Number of } \alpha \text{ particles} \times 2 \). Subtract this value from the total change in atomic number to determine the number of β particles emitted: \( \text{Number of } \beta \text{ particles} = \text{Total change in atomic number} - \text{Reduction from } \alpha \text{ particles} \).
Step 5: Verify the results. Ensure that the total change in mass number and atomic number matches the given decay series. The sum of the effects of α and β emissions should result in the transformation of Th-232 to Pb-208.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Radioactive Decay
Radioactive decay is the process by which unstable atomic nuclei lose energy by emitting radiation. This can occur through various types of decay, including alpha (α) and beta (β) decay. Understanding the decay series of an isotope, such as thorium-232, is crucial for determining the types and quantities of particles emitted during the decay process.
Recommended video:
Guided course

Measuring Radioactivity Concept 1
Alpha and Beta Particles
Alpha particles consist of two protons and two neutrons, making them relatively heavy and positively charged. They are emitted during alpha decay, which typically occurs in heavy elements. Beta particles, on the other hand, are high-energy, high-speed electrons or positrons emitted during beta decay, which involves the transformation of a neutron into a proton or vice versa. The balance of these emissions is essential for understanding the decay chain.
Recommended video:
Guided course
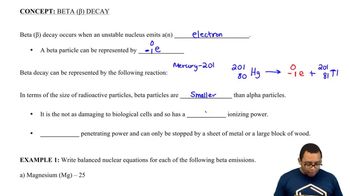
Beta Decay Concept 2
Decay Series
A decay series is a sequence of decays that a radioactive isotope undergoes until it reaches a stable end product. In the case of thorium-232, it undergoes a series of transformations through various intermediate isotopes before ultimately decaying into lead-208. Each step in the series can involve the emission of alpha or beta particles, and analyzing the total emissions requires tracking each decay event throughout the series.
Recommended video:
Guided course

Beta Decay Example 2
Related Practice
Textbook Question
2087
views
Textbook Question
Harmful chemical spills can often be cleaned up by treatment with another chemical. For example, a spill of H2SO4 might be neutralized by addition of NaHCO3. Why is it that the harmful radioactive wastes from nuclear power plants cannot be cleaned up as easily?
1363
views
Textbook Question
Why is a scintillation counter or Geiger counter more useful for determining the existence and source of a new radiation leak than a film badge?
1469
views
Textbook Question
Californium-246 is formed by bombardment of uranium-238 atoms. If four neutrons are formed as by-products, what particle is used for the bombardment?
1634
views
