Textbook Question
Locate aluminum in the periodic table and give its group number and period number.
1419
views

 Verified step by step guidance
Verified step by step guidance

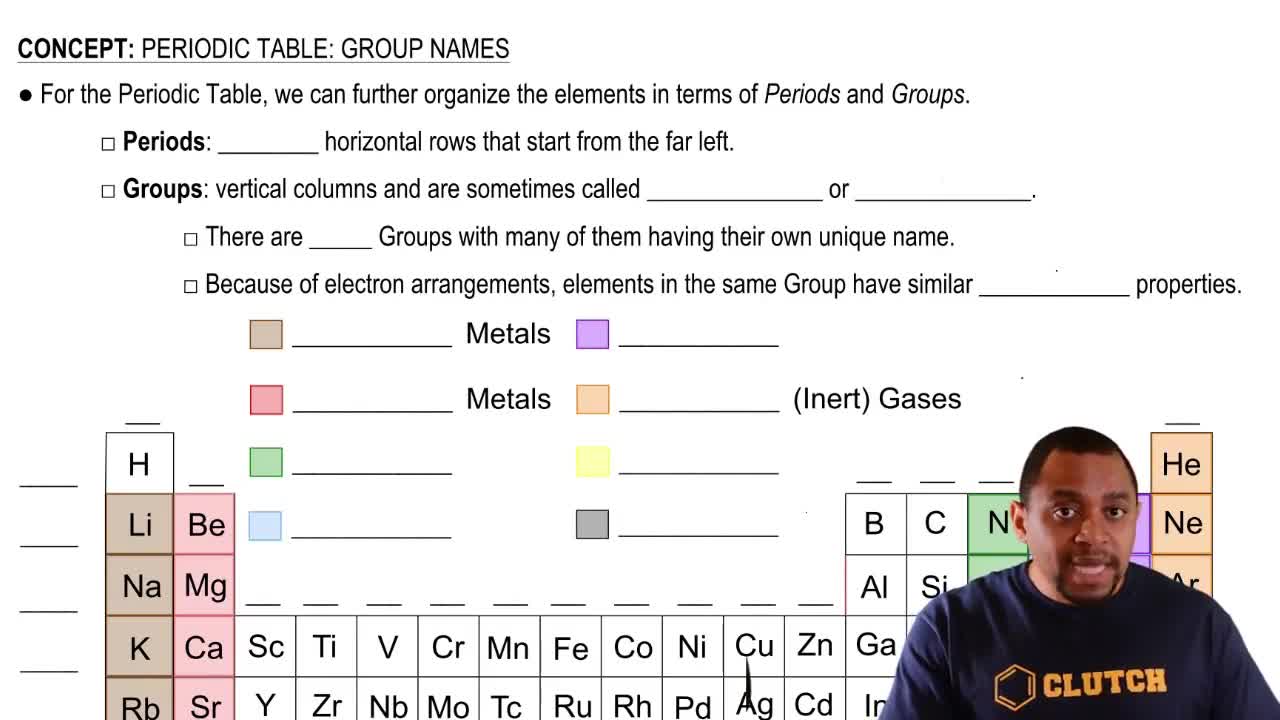
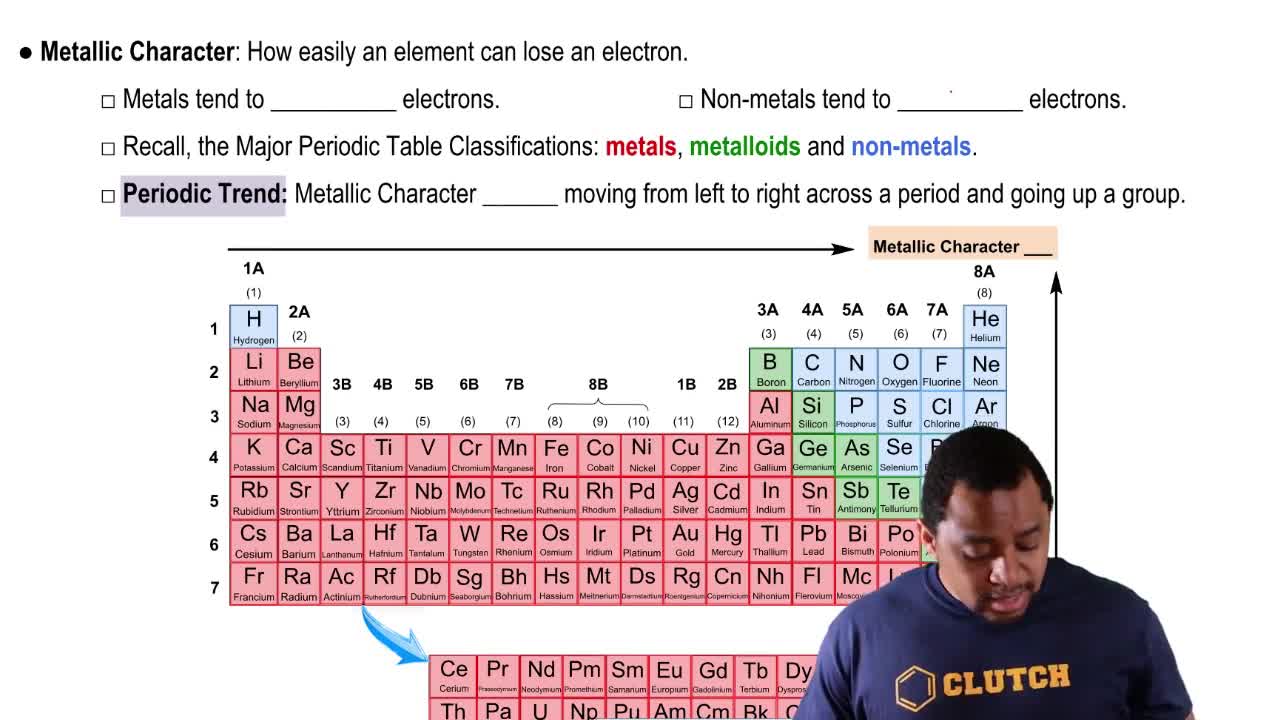
Locate aluminum in the periodic table and give its group number and period number.
How many electrons are present in an atom in which the first and second shells and the 3s subshell are filled? Name the element.
For chlorine, identify the group number, give the number of electrons in each occupied shell, and write its valence-shell configuration.
Use the following blank periodic table to show where the elements matching the following descriptions appear.
a. Elements with the valence-shell electron configuration ns2 np5
b. An element whose third shell contains two p electrons
c. Elements with a completely filled valence shell
Use the following orbital-filling diagram to show the electron configuration for As: