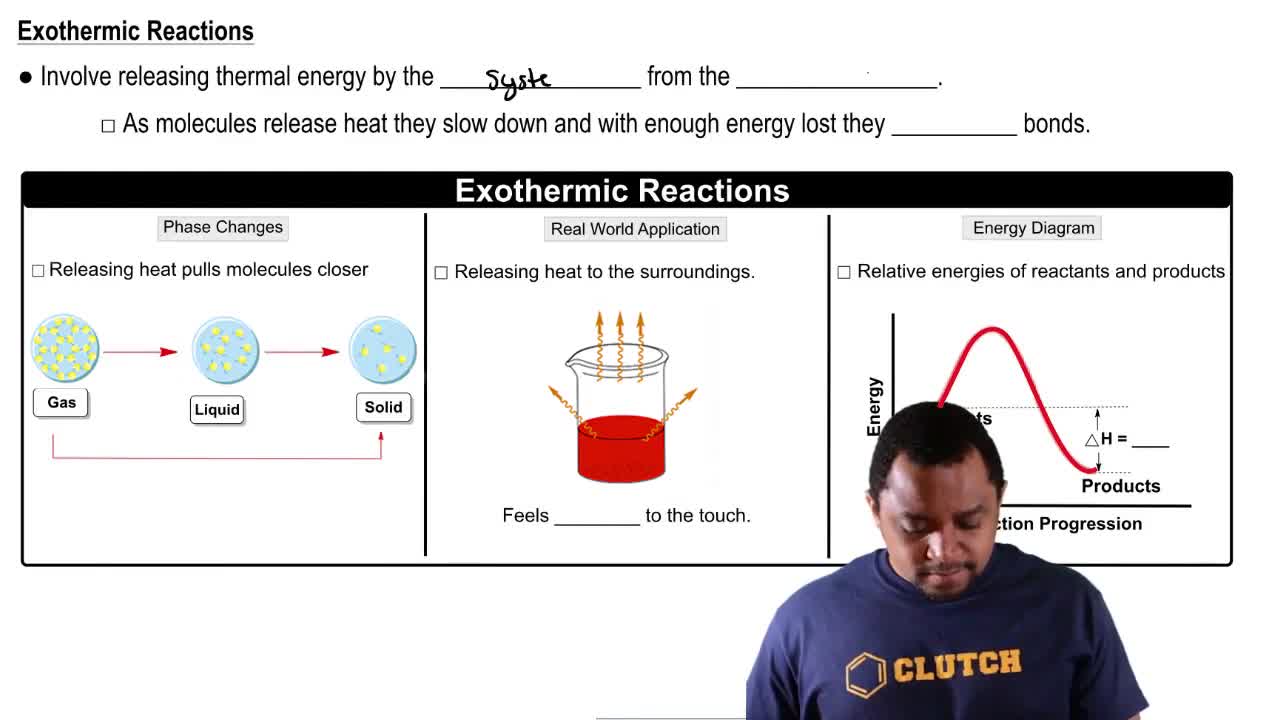
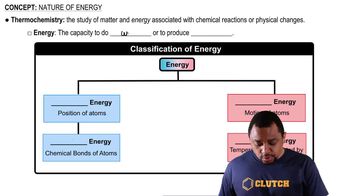
Textbook Question
Which of the following processes results in an increase in entropy of the system?
a. A drop of ink spreading out when it is placed in water
b. Steam condensing into drops on windows
c. Constructing a building from loose bricks
880
views