Textbook Question
For each of the following processes, specify whether entropy increases or decreases. Explain each of your answers.
a. Assembling a jigsaw puzzle
947
views

 Verified step by step guidance
Verified step by step guidance
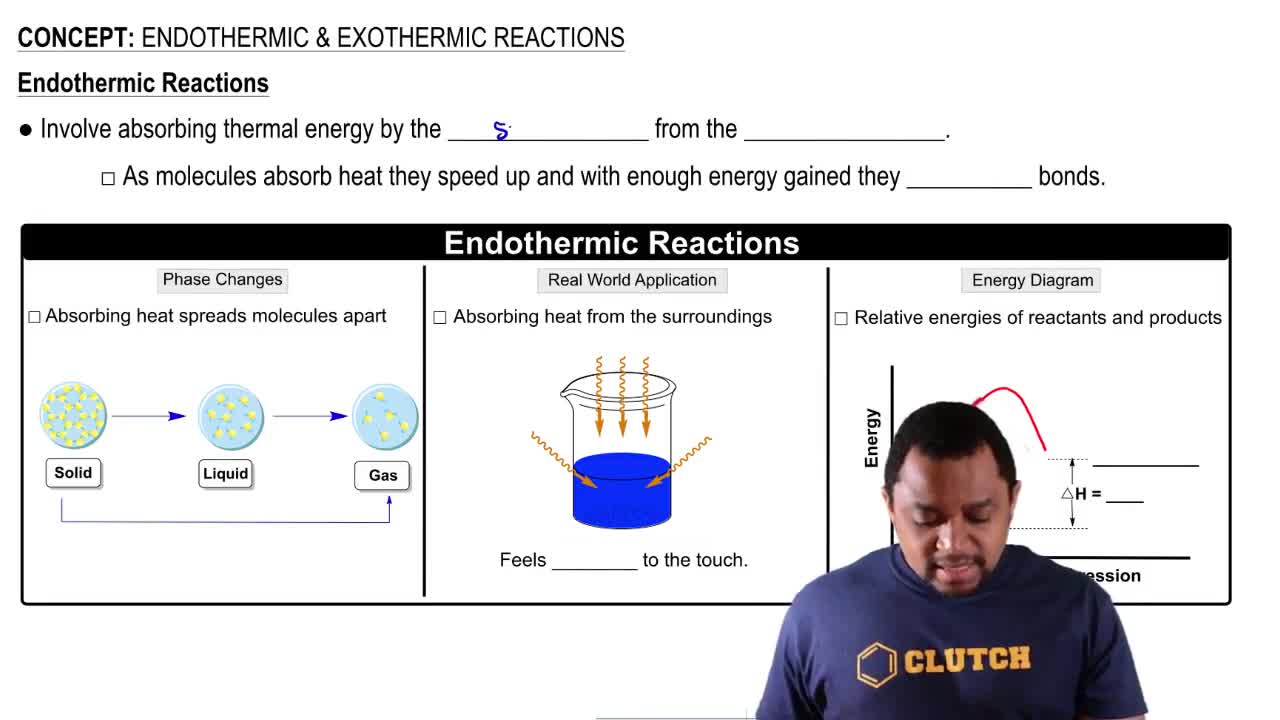


For each of the following processes, specify whether entropy increases or decreases. Explain each of your answers.
a. Assembling a jigsaw puzzle
What two factors affect the spontaneity of a reaction?
What is the difference between an exothermic reaction and an exergonic reaction?
For the reaction
b. Does entropy increase or decrease in this process?
For the reaction 2 Hg(l) + O2(g) → 2 HgO(s), ∆H = –43 kcal/mol (–180 kJ/mol).
a. Does entropy increase or decrease in this process? Explain.
For the reaction 2 Hg(l) + O2(g) → 2 HgO(s), ∆H = –43 kcal/mol (–180 kJ/mol).
b. Under what conditions would you expect this process to be spontaneous?