Textbook Question
What is meant by partial pressure?
2316
views

 Verified step by step guidance
Verified step by step guidance
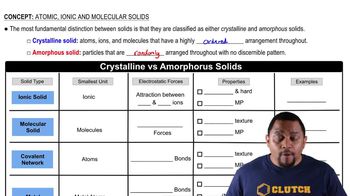


What is meant by partial pressure?
What is a liquid's heat of vaporization?
What is the effect of pressure on a liquid's boiling point?
Use the kinetic–molecular theory to explain why gas pressure increases if the temperature is raised and the volume is kept constant.
Obtain phase diagrams for water and carbon dioxide.
a. Based on the phase diagram for water, explain how it is possible to skate on ice, that is, solid water.
Obtain phase diagrams for water and carbon dioxide.
b. Would it be possible to skate on 'dry ice,' that is, solid CO2?