The structure of vinyl acetate is shown below (the partial structure H2C=CH-is known as a vinyl group). When polymerized it produces poly(vinyl acetate), a polymer used for the springy soles in running shoes. Draw the structure of the polymer obtained if three vinyl acetate units underwent polymerization.
Ch.13 Alkenes, Alkynes, and Aromatic Compounds

Chapter 13, Problem 27
Draw the product from reaction of the following substances with (1) Br2 and FeBr3 and (2) SO3 and H2SO4 catalyst (red=O):
(a) <IMAGE>
(b) <IMAGE>
 Verified step by step guidance
Verified step by step guidance1
Step 1: Recognize the type of reactions involved. The reaction with Br2 and FeBr3 is an electrophilic aromatic substitution (halogenation), where a bromine atom is introduced into the aromatic ring. The reaction with SO3 and H2SO4 is also an electrophilic aromatic substitution (sulfonation), where a sulfonic acid group (-SO3H) is introduced into the aromatic ring.
Step 2: Analyze the structure of the given aromatic compounds (a) and (b). Identify any substituents already present on the aromatic ring, as these will influence the position of the incoming groups (bromine or sulfonic acid) due to their activating or deactivating effects and their ortho/para or meta-directing nature.
Step 3: For reaction (1) with Br2 and FeBr3, determine the most likely position for bromination based on the substituents on the aromatic ring. Activating groups (e.g., -OH, -CH3) direct bromine to the ortho and para positions, while deactivating groups (e.g., -NO2, -COOH) direct bromine to the meta position.
Step 4: For reaction (2) with SO3 and H2SO4, determine the most likely position for sulfonation. Similar to bromination, the substituents on the aromatic ring will dictate the position of the sulfonic acid group (-SO3H) based on their directing effects.
Step 5: Draw the final products for both reactions (1) and (2) for each compound (a) and (b). Ensure that the substituents are placed in the correct positions on the aromatic ring based on the directing effects of the existing groups and the type of reaction.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution (EAS) is a fundamental reaction in organic chemistry where an electrophile replaces a hydrogen atom on an aromatic ring. This process is crucial for understanding how aromatic compounds react with electrophiles like bromine (Br2) in the presence of a catalyst such as FeBr3, which enhances the electrophilicity of bromine, facilitating the substitution reaction.
Recommended video:
Guided course
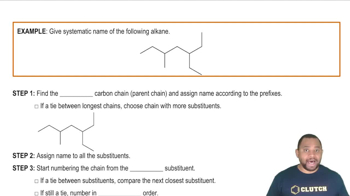
Naming Alkanes with Substituents Example 1
Sulfonation
Sulfonation is a specific type of electrophilic aromatic substitution where a sulfonyl group (SO3H) is introduced into an aromatic compound. This reaction typically involves sulfur trioxide (SO3) and concentrated sulfuric acid (H2SO4) as a catalyst, which generates the electrophile needed for the substitution. Understanding this process is essential for predicting the products of reactions involving aromatic compounds and sulfonating agents.
Reaction Mechanism
A reaction mechanism outlines the step-by-step sequence of events that occur during a chemical reaction. In the context of EAS and sulfonation, it includes the formation of the electrophile, the attack on the aromatic ring, and the subsequent loss of a proton to restore aromaticity. Grasping the mechanism is vital for predicting the outcome of the reactions and understanding the stability and reactivity of the intermediates formed.
Recommended video:
Guided course

Alcohol Reactions: Dehydration Reactions Concept 1
Related Practice
Textbook Question
66
views
Textbook Question
Draw structures corresponding to the following names (refer to Table 13.2 if necessary):
a. m-Chloronitrobenzene
b. o-Nitrotoluene
c. p-Methylaniline
d. p-Nitrophenol
813
views
Textbook Question
Reaction of Br2 and FeBr3 with phenol can lead to three possible substitution products. Show the structure of each and name them.
1712
views
Textbook Question
Alkynes undergo hydrogenation to give alkanes, just as alkenes do. Draw and name the products that would result from hydrogenation of the alkynes shown in Problem 13.25.
<IMAGE>
660
views
Textbook Question
What do the terms saturated and unsaturated mean?
1663
views
Textbook Question
Draw an example of a saturated four carbon compound and an unsaturated four carbon compound.
871
views
