Textbook Question
Draw structures corresponding to the following names (refer to Table 13.2 if necessary):
a. m-Chloronitrobenzene
b. o-Nitrotoluene
c. p-Methylaniline
d. p-Nitrophenol
813
views

 Verified step by step guidance
Verified step by step guidance


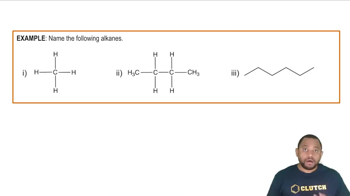
Draw structures corresponding to the following names (refer to Table 13.2 if necessary):
a. m-Chloronitrobenzene
b. o-Nitrotoluene
c. p-Methylaniline
d. p-Nitrophenol
Reaction of Br2 and FeBr3 with phenol can lead to three possible substitution products. Show the structure of each and name them.
Draw the product from reaction of the following substances with (1) Br2 and FeBr3 and (2) SO3 and H2SO4 catalyst (red=O):
(a) <IMAGE>
(b) <IMAGE>
What do the terms saturated and unsaturated mean?
Draw an example of a saturated four carbon compound and an unsaturated four carbon compound.
What does the term 'aromatic' refer to when discussing organic molecules?