Textbook Question
Ethers have some slight solubility in water. Explain this using the concept of hydrogen bonding.
39
views

 Verified step by step guidance
Verified step by step guidance

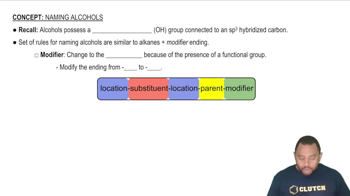

Ethers have some slight solubility in water. Explain this using the concept of hydrogen bonding.
For each of the following molecules, (i) redraw using line structure format, (ii) identify its hydrophobic and hydrophilic parts, and (iii) predict its solubility in water.
c.
What alkenes might be formed by dehydration of the following alcohols? If more than one product is possible in a given case, indicate which is major.
b.
What alcohols yield the following alkenes as the major product on dehydration?
b.